Abstract
Background
Ponatinib is currently licensed for the treatment of patients resistant or intolerant to at least 2 tyrosine kinase inhibitors (TKI) or in patients harboring a T315I ABL mutation, in chronic, accelerated and blast phase chronic myelogenous leukemia (CML) or Ph+ ALL. Surprisingly, very little is known in terms of pharmacokinetics of this drug in CML patients and their impact on outcomes.
Aims
We evaluated the impact of Ponatinib plasma concentrations determinations in chronic phase CML (CP CML) patients during treatment, and outcomes.
Methods
We enrolled after informed consent patients in CP CML in 3 cooperative centers, receiving Ponatinib at any dose and measured the plasma concentration of this compound at any time during treatment by UPLC with mass tandem detection. Data were expressed as µg/L and 24H concentrations (C24h, expressed in mg/kg/d). The C24h were estimated according to the average half-life calculated for the entire cohort. After informed consent, basic clinical data, hematologic, cytogenetic and molecular results were captured retrospectively as well as outcome. qRT-PCR results were expressed as BCR-ABL ratios in %, standardised according to the international scale, with at least 32,000 copies of ABL as control.
Results
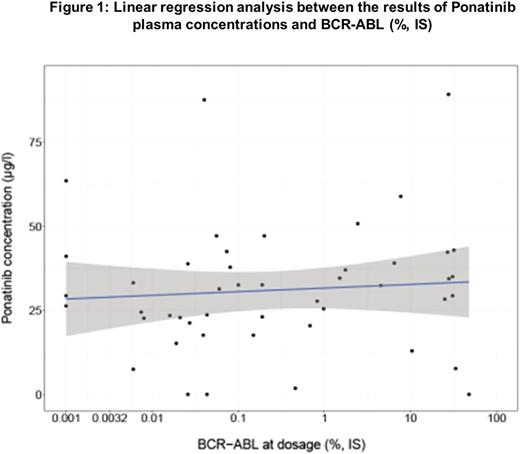
Thirty-seven CP CML patients have been involved in this study, and 50 blood samples were studied. There were 20 males and 17 females of a median age at diagnosis of 44.6 (7.6-82.5) and of 55.7 (25-86) years at ponatinib plasma measurement. Sokal score was low for 18%, intermediate for 12% and high for 70% of patients (9 patients unknown). One patient had a variant Ph, one a cryptic Ph and all the others a classical form of the Ph chromosome. All patients had a "major" BCR transcript except 1 (e19a2).The median BCR-ABL at diagnosis was 66.5 (33-110) % (IS). The median follow-up since diagnosis was 8.65 (2.5-16.7) years, 8.57 (2.48-16.7) years since first TKI initiation, and 4.32 (0.77-6.1) years since Ponatinib initiation. Two patients received Ponatinib as first-line (EPIC trial). Three patients received Ponatinib as second line, 12 as third line and 20 as fourth line therapy, all for resistance to previous lines of TKI. Fourteen patients (38%) were ABL mutated (10 T315I, 1 V379I, 1 Q252H, 1 L298V, 1 E255K). The median dose at Ponatinib initiation was 45 (30-45) mg daily and at Ponatinib plasma measurement at 30 (5-45) mg daily which represented a median C24h of 0.41 (0.05-0.94) mg/kg/day. The blood test was performed after a median of 12.25 (0.5-92.5) hours after last oral dose. Median plasma concentrations of Ponatinib were 30.75 (0-89.1) mg/l. When considering a target therapeutic concentration of 20 mg/l (= 40 nM, Cortes J. et al. NEJM 2013), 39 patients were above this level and 11 below. At plasma determination, 9 patients were in CHR, 1 in minimal CyR, 1 in minor CyR, 7 in partial CyR, 14 were in CCyR, 13 in MMR, 3 in MR4, and 2 in MR5. The median BCR-ABL at that time was 0.15 (0-47.9)% (IS). There was no significant correlation between the response observed and the plasma concentrations measured in patients and their molecular response (Figure 1.), Spearman correlation test: r= 0.134, p=0.34.
There was no correlation neither with the onset of vascular events but the blood sampling was not necessarily performed when these events occurred (n=8). However and indeed, this population might be highly selected, as the majority of the patients without severe side effects and responding to treatment are maintained on Ponatinib. At latest follow-up, 4 patients died in this heavily pre-treated population of patients, 2 from post-allogeneic infectious complications, 1 form a stroke, 1 from a metastatic spinocellular carcinoma.
Conclusion
The determination of TKI plasma levels is surely useful in screening patients for uncompliance (here one patient), however their relationship to the efficacy or toxicity of such a compound remains to be further assessed in a more homogeneous and larger population of patients.
Nicolini: Incyte Biosciences: Honoraria, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau. Rea: Pfizer: Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal